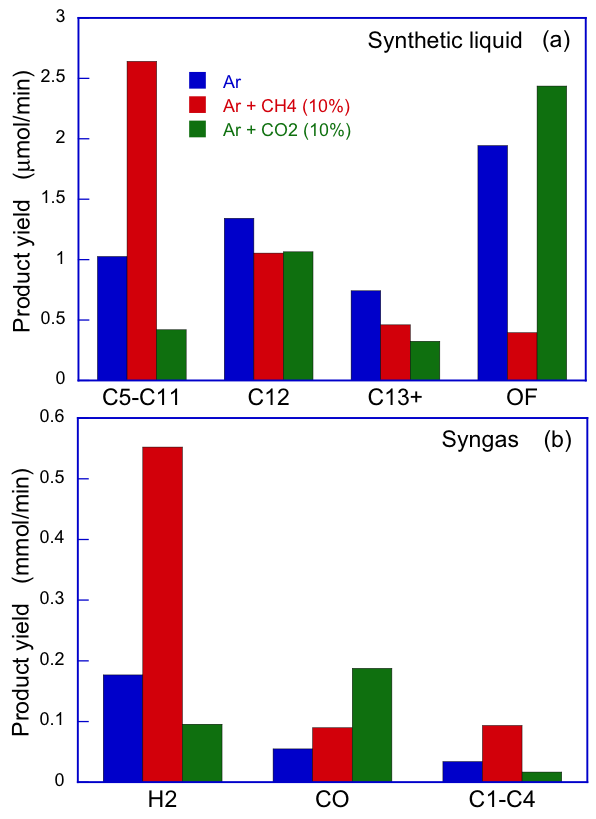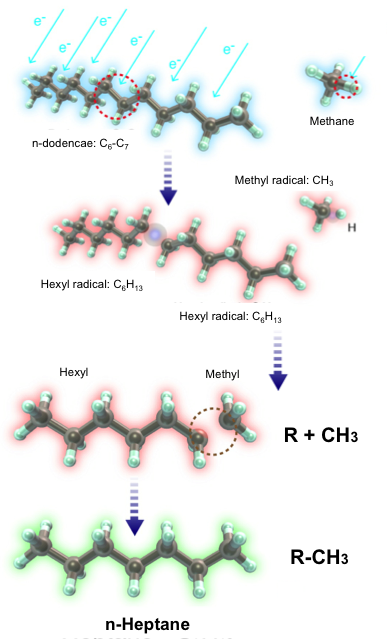
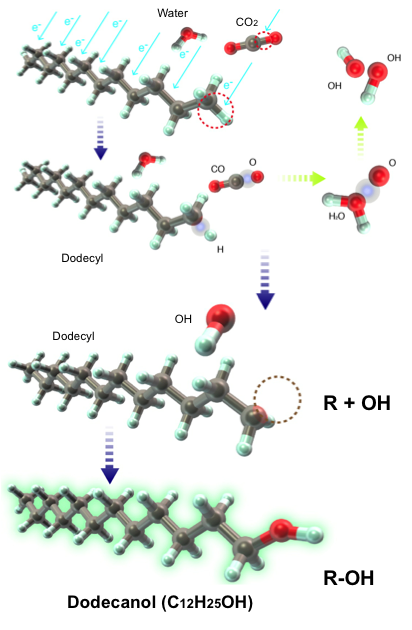
We invented an original technical approach to simultaneously produce a tailored synthetic liquid fuel and a syngas. In an aqueous discharge reactor with gaseous bubbles, we reformed an emulsified n-dodecane/water mixture. The higher dielectric permittivity of the mixture facilitates electrical discharges that cause the electron impact dissociation of n-dodecane into alkyl and hydrogen radicals, while the addition of water also provides a steam-reforming environment inside the discharged bubbles. We added methane and carbon dioxide to the system because they dissociate into methyl and oxygen radicals, respectively, which prevent the alkyl-alkyl recombinations that result in the formation of long-chain hydrocarbons (HCs). Thus, we were able to control product selectivity by adding methane to increase the production of short-chain HCs and hydrogen gas or by adding carbon dioxide to increase the production of oxygenated fuels, such as 1-dodecanol. Using gas chromatography and gas chromatography-mass spectrometry we detail the compositions of both the synthetic liquid and the syngas, and we provide conceptual chemical mechanisms to selectively increase the production of oxygenates and that of HCs that are shorter or longer than the base fuel. The basis of this in-liquid discharge for the purpose of fuel reforming has potential applications to advanced engines to control ignition delay time, a continuing focus of study in our lab.