

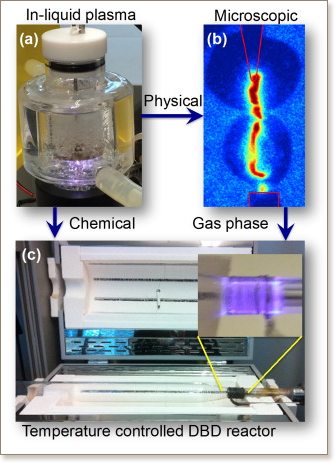
This paper presents experimental results for reforming n-heptane in a temperature-controlled dielectric barrier discharge reactor to show detailed chemical composition in the products and to propose a potential method to control the product composition. Reformed products of n-heptane and water mixture in an inert Ar feed could be identified as hydrogen, carbon monoxide, oxygenates, and various hydrocarbons, having a wide range of carbon numbers. To selectively increase production of short-chain hydrocarbons, Ar was replaced by CH4. An increased pool of methyl radicals, via plasma chemistry of CH4, might facilitate to stabilize intermediate alkyls (R) into RCH3, which successfully increased short-chain hydrocarbon concentration. When CO2 was supplied instead of Ar (to provide enriched OH and O radicals), significantly higher oxygenate concentrations were obtained through the stabilization of alkyls as ROH (alcohol), and RC(double bond; length as m-dashO)R′ (ketone). The use of methane and carbon dioxide as feed to tailor the products of plasma-assisted reforming of n-heptane with methyl (CH3), or O radicals, is successfully demonstrated in the presence of water vapor. Detailed product analysis, such as product selection, rates and energy efficiency using a gas chromatograph and a gas chromatography mass spectrometer, will be elaborated upon.